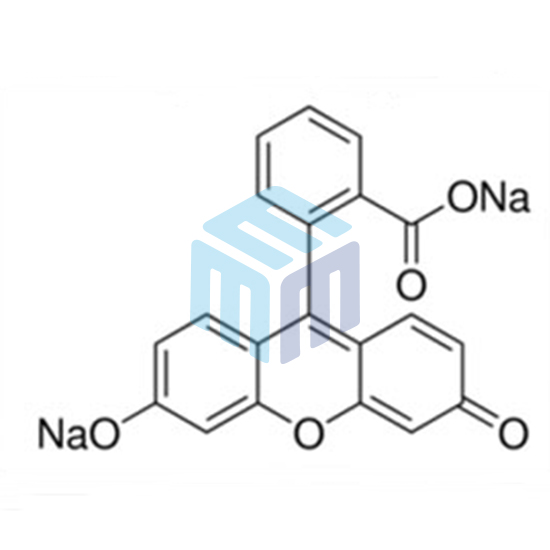
Fluorescein Sodium USP
Inquiry NowProduct Description
PRODUCT SPECIFICATIONS
| Name Of Product: | Fluorescein Sodium USP |
| Pharmacopoeial Name: | Fluorescein Sodium USP |
| CAS No: | 518-47-8 |
SPECIFICATIONS
| Sr. No | Criteria | Limit/Specification |
| 1. | APPEARANCE | Orange Red, Fine powder, Hygroscopic |
| 2. | IDENTIFICATION | Must Comply Identification Test A to c |
| 3. | ACRIFLAVINE | No precipitate found |
| 4. | ZINC | No turbidity found |
| 5. | ORGANIC IMPURITIES (HPLC) | 1. RESORCINOL (Impurity A) – NMT 0.5 % 2. PHTHALIC ACID (Impurity B) – NMT 0.5% 3. FLUORESCEIN RELATED COMPOUND C (Impurity C) – NMT 0.5% 4. UNSPECIFIED IMPURITIES (Other Impurity) – NMT 0.1% Unspecified impurity (RT 11.7 Mint.) Unspecified impurity (RT 14.5 Mint.) 5. TOTAL UNSPECIFIED IMPURITIES (Sum of impurities) – NMT 0.5% |
| 6. | WATER DETERMINATION | NMT 17.0% |
| 7. | ASSAY ( On Anhydrous Substance) | 90.0% to 102.0% |
| 8. | Additional test: Pyrogen Free test | Must Pass Test |
RESIDUAL SOLVENTS
| 9. | Acetone | ≤5000 PPM |
MICROBIOLOGICAL QUALITY
| Sr. No | Criteria | Limit/Specification |
| 1. | TAMC | ≤100 CFU/g |
| 2. | TYMC | ≤ 50 CFU/g |
| 3. | Bile tolerant Gram-Bacteria | Absent/g |
| 4. | Staphylococcus Aureus | Absent/g |
| 5. | Pseudomonas Aeruginosa | Absent/g |
| 6. | Escherichia Coli | Absent/g |
| 7. | Salmonella | Absent/g |
We are a USA company catering products and solutions in the USA, Australia, New Zealand, India, Singapore, Malaysia, South Korea, Indonesia, Dubai, Philippines and Vietnam. Our team of experts across different platforms can discuss and customize your requirements as per your needs.
Please reach out to us at info@mkubeenterprise.com or call us at +1-732-808-1999 to discuss your projects.